23 Dec VOLITILITY – A Closer Look
Volatility is a lubricant’s evaporative loss. The more volatile a lubricant, the lower the temperature at which it begins to evaporate. The more it evaporates, the less oil is left to protect equipment and the faster it must be replace.
Small, light molecules in conventional lubricants evaporate at relatively low temperatures. These light molecules require less energy in the form of heat than heavier molecules to be lifted out of the solution and into the air. The tendency of a liquid to evaporate is volatility.
Why is Volatility Important?
Volatility is a common phenomenon and many drivers have experienced it when their vehicles “use” motor oil in irregular intervals. Some vehicles seem to use oil rapidly soon after an oil change, but stabilize after a short time when makeup oil is added. This is caused by the lighter elements evaporating out of the solution, causing the oil level to drop after the initial oil change. Adding oil to replace this loss leads to stabilization since the majority of light elements are now gone.
Volatility affects more than the rate of oil consumption. When light elements in oil evaporate from heat, the oil’s viscosity increases. This thicker oil forces the engine to work harder, resulting in several problems:
- Performance loss
- Fuel-economy loss
- Poor cold-temperature starting
- Increased engine deposits
Volatility causes oils to thicken with use, oil becomes harder to pump. Pumps that must move thicker oil wear more quickly and consume more energy.
Parts require more energy to move through thicker oil than in thinner oil. As a result, extra energy is spent on pumping and moving through thick oil, reducing performance and fuel economy.
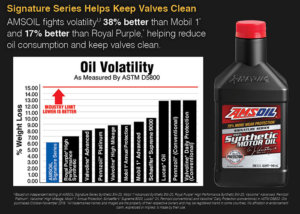
Noack Volatility Test (ASTM D5800)
The most common method used in measuring oil volatility is the Noack Volatility Test. In this test, an oil sample is weighed and then heated to a temperature of 482°F (250°C) for one hour. During this time, dry air is passed over the sample, which carries off the oil vapors that have boiled off and deposits them in a beaker attached to the apparatus. Finally, the original sample is removed and re-weighed. Any reduction in weight is reported as a percentage loss of the original weight. The entire procedure is similar to the operation of a petroleum fractioning tower or still.
API SP and ILSAC GF-6 performance classifications require volatility weight loss be no greater than 15 percent for all viscosity grades.
Understanding Flash and Fire Points
Flash and fire points help describe a lubricant’s high-temperature performance and stability.
The flash point is the lowest temperature at which the vapor above an oil sample ignites when a flame is passed over it. Once the flame on the surface of the oil continues to burn for at least five seconds after the ignition flame has been removed, the temperature is recorded as the oil’s fire point. Fire points are generally higher than flash points by 10°F to 40°F (5.6°C to 22.2°C). An additional classification, the auto-ignition point, is the temperature at which oil ignites on its own without the aid of an outside ignition source.
It’s important to note that flash and fire points should not be used to ascertain an oil’s usable temperature range. This range is typically 100°F to 150°F (37.8°C to 65.5°C) lower than reported flash- and fire-point values.
Flash and fire points can be significantly different between lubricants. Some lubricants have a relatively small temperature range among flash, fire and autoignition points, while others have a significantly larger range. Oils that are more stable tend to have flash and fire points that are higher and closer together than oils that are more volatile.
Conventional lubricants often contain chemicals that break down at normal operating temperatures. The presence of oxygen increases the likelihood of chemical breakdown, and oxygen can be found in almost all vehicle and equipment systems.
When contaminants in conventional oils break down, they deposit sludge and varnish on component surfaces, which leaves the oil thick and hard to pump. Oil that has broken down also has little heat-transfer capability.
High flash and fire points tend to suggest improved high-temperature stability, which reduces oil consumption and increases the oil’s service life.
The Cleveland Open Cup Test (ASTM D92)
The Cleveland Open Cup Test (ASTM D92) measures motor oil flash and fire points. This test is intended for fluids having a flash point of 175°F (79.4°C) and above. A fixed volume of fluid is heated at a uniform rate while open to the atmosphere at its surface. A small flame is passed over the surface at uniform temperature increments to determine the point at which vapors ignite. This temperature is recorded as the oil’s flash point. At a somewhat higher temperature, self-sustained burning for at least five seconds determines the point.
AMSOIL Advantage
Less Volatility
AMSOIL synthetic lubricants are engineered to have uniform molecular shapes and weights. The advantage is that there are fewer “light fractions” that are susceptible to evaporation. AMSOIL synthetic lubricants are more stable than conventional motor oils for improved resistance to burn-off.
High Flash and Fire Points
AMSOIL synthetic lubricants display high flash and fire points, which are highly resistant to breakdown at normal operating temperatures. Amsoil offers more protection than conventional oils because they resist oxidation and thermal breakdown, retaining their pumpability and heat-transfer abilities.